The relative atomic mass of an atom is the average mass of one atom of that element compared to 1/12 of the mass of one carbon-12 atom. Basically, it is not practical for scientists to use actual masses of atoms in scientific calculations since atoms have very small masses.
Related Topics: More Chemistry Lessons- Relative mass valuation is a quick way to go through a large backlog of stories and estimate them all as they relate to each other. To use this approach, first write up a card for each story.
- Relative atomic mass meaning: 1. The mass of an atom of a particular chemical element, usually expressed in atomic mass units 2.
- how to calculate the relative formula mass or relative molecular mass
- how to use the relative molecular mass to calculate the percent mass of an element in a compound
- how to use the relative molecular mass to calculate the percent mass of water in a compound
The following diagram shows how to calculate the relative molecular mass or relative formula mass. Scroll down the page for more examples and solutions.
What is relative formula mass and relative molecular mass?
The relative formula mass of a substance is the sum of the relative atomic masses of the elements present in a formula unit. The symbol for relative formula mass is Mr.
If the substance is made of simple molecules, this mass may also be called the relative molecular mass.
Example:
What is the relative mass formula of hydrogen gas? (Relative atomic mass: H = 1)
Solution:
The formula for hydrogen gas is H2. Each molecule contains 2 hydrogen atoms.The relative mass formula of hydrogen gas is
Mr(H2) = 2 × Ar(H) = 2 × 1 = 2
Example:
What is the relative mass formula of water? (Relative atomic masses: H = 1, O = 16)
Solution:
The formula for water is H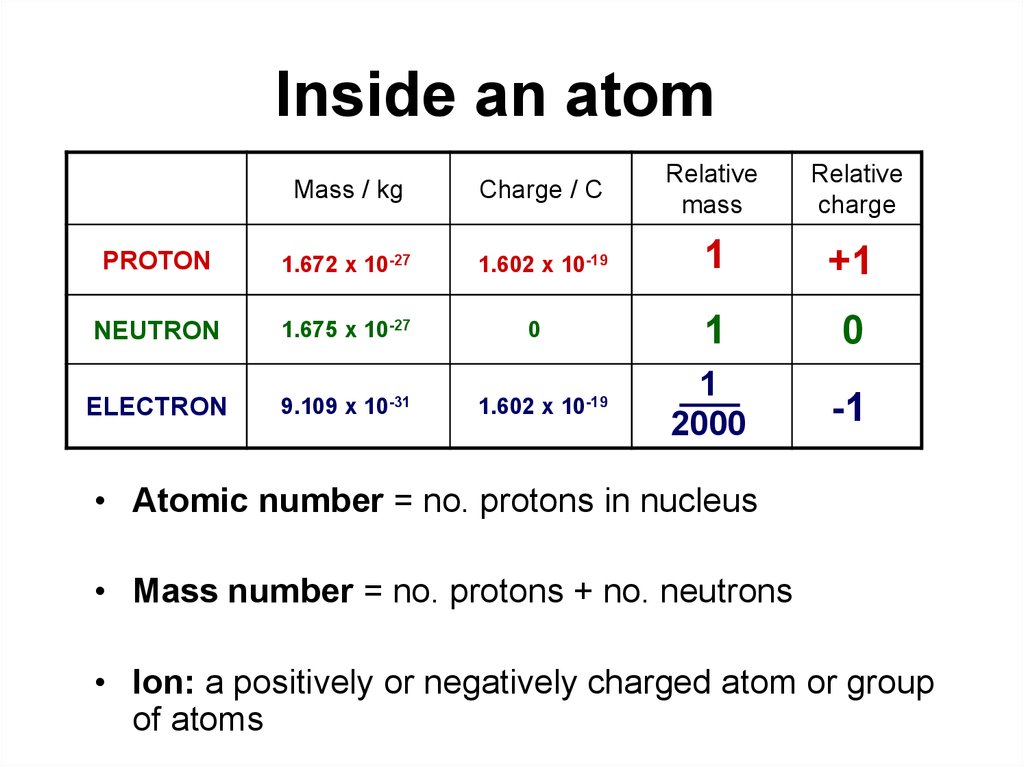 2O. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.
2O. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.The relative mass formula of water is
Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Example:
What is the relative mass formula of sodium chloride? (Relative atomic masses: Na = 23, Cl = 35.5)
Solution:
Sodium Chloride is an ionic solid with the formula Na+Cl-.The relative mass formula of sodium chloride is
Mr(NaCl) = Ar(Na) + Ar(Cl) = 23 + 35.5 = 58.5
How to find the Percent Mass of Elements in a compound?
How to use Mr to calculate the percent mass of an element in a compound?
Example:
What percentage of the mass of ammonium nitrate is nitrogen? (The formula for ammonium nitrate is NH4NO3, Relative atomic masses: H = 1, O = 16, N = 14)
Solution:
Mr(NH4Relative Masse
NO3) = (2 × 14) + (4 × 1) + (3 × 16) = 28 + 4 + 48 = 80Mass of nitrogen in the formula = 28
Mass of nitrogen as a fraction of the total =
Mass of nitrogen as percentage of total mass
Example:
What percentage of the mass of O in NaNO3? (Relative atomic masses: Na = 23, O = 16, N = 14)
Solution:
Mr(NaNO3) = 23 + 14 + (3 × 16) = 23 + 14 + 48 = 85
Relative Mass Of Subatomic Particles
Mass of O in the formula = 48Mass of O as a fraction of the total =
Mass of O as percentage of total mass
How to find percent by mass and percent composition?
Example:
Find the percent composition by mass of potassium dichromate (K2Cr2O7)
Step 1: Find the molar mass of the compound.
Step 2: Divide the total mass of each element by the molar mass and multiply by 100 to find the % mass.
- Show Step-by-step Solutions
Finding the percent by mass means finding the mass of the elements in the compound and adding the masses for the total mass.
Example:
You have a 7364 milligrams sample of SO2. How many grams of sulfur are in the sample?
How to calculate the Mass Percent of an Element in a Compound?
Mass Percent Composition of an Element in a Compound
To calculate the mass percent composition (or simply, the mass percent) of an element in a compound, we divide the mass of the element in 1 mol of the compound with the mass of 1 mol of the compound and multiply by 100%.
Examples:
1. What is the mass percent of carbon in carbon dioxide?
2. What is the mass percent of oxygen in carbon dioxide?
- Show Step-by-step Solutions
How to use Mr to calculate the percent mass of water in a compound?
Example:
What percentage of the mass of magnesium sulfate is water? (Given that the formula for magnesium sulfate is MgSO4•7H2O, Relative atomic masses: H = 1, O = 16, S = 32, Mg = 24)
Solution:
Mr(MgSO4•7H2O) = 24 + 32 + (4 × 16) + (7 × 18) = 246Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Mass of water in the formula = 18 × 7 = 126
Mass of water as a fraction of the total =
Mass of hydrogen as percentage of total mass
How to calculate the Water of Crystallization?
This video outlines how to determine the percent, by mass, of water trapped in a hydrate's crystal lattice.
Example:
What is the percent of water, by mass, in the hydrate CuSO4•2H
Relative Mass And The Mole
2O How to calculate the percentage of water in the formula of a hydrated compound?Example:
Relative Mass Of Proton Neutron Electron
Determine the % water in the following hydrates:
a) CuSO4•5H2O
b) CaCl2•2H2O
c) KAl(SO4)2•12H2O
- Show Step-by-step Solutions
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
Why is the mass number an integer but the relative atomic mass is not?
1 Answer
Well the mass number refers to a given
Explanation:
A nuclide is a specific isotope, which of course has specific
On the other hand, the
By way of example, the element boron has
And so the
Related questions
