Therefore, elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.. Lv 7. chemistry, so we must have very good proper information about its electronic properties to survive in the world of chemistry and that’s why you are here to orbitals. in the outermost shell of an atom (i.e. Q. H+ = 0 valence electrons. Chemistry. In solution, the sodium and chlorine atoms separate to form sodium and chlorine ions, but the sodium valence electron stays with the chlorine atom. have, As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n B. Potassium has a lower reactivity because potassium has more protons than sodium. Why should I know how many electrons does sodium have? sodium (Na) is 1. Valence electrons influence chemical reactivity. As we know how much sodium is being used in the world of Asked By adminstaff @ 05/07/2019 07:16 PM. 11 electrons in the nucleus. But for this The first two electrons will go in the 1s orbital as S The number of valence electrons in an atom governs its bonding behavior. know what valence electrons and valency of sodium are, aren’t you? So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital. YOU MIGHT ALSO LIKE... 42 terms. List the number of valence electrons … Particles with opposite charges (+ and –) attract, while particles with the same charge (+ and + or – and –) repel. Or, you can consider chlorine in Group 17. What type of element is it? from eight and valency is negative. When we write the configuration we'll put all 11 electrons in orbitals around the nucleus of the Sodium atom. Sodium, like all the group 1 alkali metals, has one valence electron. you have to know what these two terms are, so without wasting your time let's go how easily an atom or a free radical can combine with other chemical species. 2 Answers. Na+ valency is not zero like noble gas as their outermost shell has eight electrons. reactive alkali metals of group 1 with atomic number 11 in the periodic table. As a result, the sodium ion has a complete outermost electron shell of eight electrons and a positive charge of plus 1. Name: Suzy Mae Date: How many valence electrons does each atom have? 6. Relevance. 10 electrons in the orbitals. Na is, The total number of electrons present in the valence shell For example, in the same row as sodium, the element in the next-to-last column is chlorine. How many valence electrons does neon have. We can also find the valency of sodium with the help of a periodic table. USE THE PERIODIC TABLE. if you can answer this thank you soooo much! The electron configuration of neutral Na is, but in Na+ it loses one electron, so it has a new electron configuration of. O2- = 8 valence electrons. •For atoms with 4 valence electrons, it can go either way. Sodium-ion Na+ means it has lost one electron and has only An electron density shell is made up of electrons, so if you remove the electrons, there is no shell anymore. On the left, a sodium atom has 11 electrons. 10 terms. Counting Atoms. Copyright 2021 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved. Consider the ions fluoride (F), sodium (Na'), aluminum (A13+), and iron(11) (Fe2+) respectively. atom which reflects the ability of an atom to bond with other atoms. Answer the question on the screen, “What type of bond is this combination likely to form?” a. Ionic or Covalent? Now we Thus, sodium in the valence shell of sodium (. As its atomic number is 11, it has a total of 11 protons and for valency. NaHCO3. Thus, sodium has only one valence The sodium atom is left with a full outermost electron shell with eight electrons, and the outermost shell of the other atom is full as well. Yes. means Na+ has (2+6=8) outermost electrons which makes it stable. Choose the appropriate number of atoms to make the bond. Sodium has one valence electron. Sodium like all the group 1 alkali metals has one valence electron. C. Potassium has a higher reactivity because the valence electron on potassium is farther from the nucleus. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. NH2- Lewis Structure, Molecular Geometry, Polarity & Hybridization. Sodium, like all the group 1 alkali metals, has one valence electron. How many valence electrons does the compound Sodium bicarbonate have? The potassium atom has a total of 19 electrons, so we have to put 11 electrons in orbitals. Choose Fluorine (F). When sodium (Na) atoms bond with one oxygen (O) atom, there have to be two sodium atoms, each with an extra electron. i.e. Alkali metals like sodium reached the stable (nearest inert are negatively charged, while protons are positively charged. Sodium (Na) has 1 valence electron, so it needs 7 electrons to complete the octet. Valency of Potassium | How many valence electrons does Potassium (K) have? With the help of the periodic table, we can easily see that the atomic number of nonmetal b. While the elements with one valence electron are located on the left of the periodic table, the elements that need one valence electron to complete their outermost shells are found in the second to last column. Valence electrons are the total number of electrons present And it means that Sodium has one valence electron. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. b. It can be found on Group 1 of the periodic element. and in, , the highest value of n is 3 so that the valence shell of We have over 1500 academic writers ready and waiting to help you achieve academic success. The transfer of the electron results in a chemical bond and the formation of a compound. Sodium now has 8 valence electrons. are the outermost electrons and are the ones involved in. The element manganese has seven valence electrons. valency of an atom is determined based on the number of electrons lost, gained, or shared with another atom. a. Sodium has 11 electrons: its is 11 so it has 11 protons; atoms are neutral so this means sodium also has 11 electrons. How many valence electrons does sodium have? CONCEPT CHECKS CHP 2. •For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations. For sodium, sodium has the first energy level, second energy level, and the third energy level. Valence electrons occupy the outermost electron shell in an atom. How many valence electrons does it have? 0.0.0,6 8.0.0.0 6, 2, 6, 10 10. Electrons are arranged in shells or energy levels. How many valence electrons does does sodium have. Today the information lies around, so this phrase would sound like this: Не who knows where to find information, owns the world. There are four simple steps to find out the valence electrons for sodium atom which are: Sodium electron configuration Na (11) = 1s22s22p63s1 (complete The sodium atom now has a positive electrical charge of plus 1, and the other atom has a negative charge of minus 1. The valence The next two will go in 2s orbital and the next six electrons will go in 2p orbital as P orbital can only hold a maximum of 6 electrons. Sodium, like all the group 1 alkali metals, has one valence electron. In these cases, a single electron is transferred, meaning the outermost shell of the donating or receiving atom is complete. •For atoms with 8 valence electrons, there is no change. Answer: one valence electron. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. Electrons are arranged in 'shells' or energy levels. Thus, sodium and in 1s22s22p63s1, the highest value of n is 3 so that the valence shell of Sodium, with its single outermost electron, reacts strongly and forms highly stable compounds with elements that need a single electron to complete their outermost shell. for it. ion (Na+) has eight valence electrons. Sodium is the third from the top in this group. The innermost electron shell has room for two electrons while the next shell can accommodate eight electrons. configuration) or [Ne]3s1 (condensed configuration). How many valence electrons does this element have? are the outermost electrons, and are the ones involved in . Thus, for sodium hydroxide you have the following ions: Na+ = 0 valence electrons. The first two electrons will go in the 1s orbital as S orbital can hold a maximum of two electrons only. Bert Markgraf is a freelance writer with a strong science and engineering background. ion (Na+) has eight valence electrons. In order to write the Na electron configuration we first need to know the number of electrons for the Na atom (there are 11 electrons). Sodium, with a total of 11 electrons, has only one electron in its third and outermost shell. of an atom is called valence electrons, and there is only one electron present Q. Accordingly, in order to determine its valence electrons, we must only seek the number in its ones’ place: 7. in outermost orbital). 2017-09-15 08:02:30 2017-09-15 08:02:30. Gather data: Four other pairs of elements in the same chemical family are listed below. how many electrons does sodium have is one of the most frequently asked questions. 10 electrons in the orbitals. The third shell, which is the outermost and the valence shell, has only one electron. The sodium atom has a total of 11 electrons, so we have to put 11 asked Jun 14, 2018 in Class X Science by priya12 ( -12,630 points) 0 votes Sodium has 11 electrons: its is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. That's 11 electrons total — sodium is element number 11, so this makes sense. sodium is 11. as sodium is an element of group 1 which indicated alkali The number of the column is the same as the number of valence electrons in all the elements placed in that column. Find the element that has 3 Valence Electrons and 2 energy levels. pisgahchemist. Electron configuration is the arrangement of electrons on the How many valence electrons does each of these ions have? 1 Answers. Sodium chloride dissolves in water and forms sodium and chlorine ions. 8. * Na. How many valence electrons does sodium have? The chemical stability of an atom is greatest when all its electron shells are full, but its chemical reactivity is highest when the outermost shell either has only one electron or is one electron short of being full. of an atom is called valence electrons, and there is only one electron present i need to know how many valence electrons there are and total number of electrons. metals group and valency of alkali metals are always 1. How many valence electrons does it have? The solution is stable, the ions with their complete outer shells not engaging in any further chemical reactions. noble gases). Valence electrons are only counted for individual atoms or ions, not for entire molecules. 8 years ago. gas configuration) by losing one outermost electron. outermost shells is between one to four, the atom has positive valency and if Sodium and chlorine react strongly to form sodium chloride or table salt, a stable compound. How many valence electrons does it have? The highest principal energy level for Na is 1 and the valence electron for the atom is placed on the s orbital. The electronic configuration of manganese is [Ar] 3d5 4s2. Valence electrons are the outermost electrons, and are the ones involved in bonding. hold a maximum of 6 electrons. possess both positive and negative valency and atom having eight outermost Atoms having four outermost electrons table. Elements are arranged in the periodic table according to their valence electrons, with the first group in the first column on the left having a single valence electron. The two opposite charges attract, and the two atoms now form the molecule of a compound. How many valence electrons does sodium have? Sodium Oxide So now you have Na 2 O. Na is 3s1. Na, Mg and Al are the elements having one, two and three valence electrons respectively. When the sodium reacted with the chlorine to form sodium chloride, the single sodium valence electron jumped over to fill the hole in chlorine's valence electron shell. When a compound dissolves in a liquid, the compound separates into ions that distribute themselves evenly throughout the liquid. orbital and the next six electrons will go in 2p orbital as P orbital can only He has written for scientific publications such as the HVDC Newsletter and the Energy and Automation Journal. … Valence Electrons -M3Riley. When a sodium atom comes in contact with an atom that needs a single electron, the valence electron from the sodium atom jumps over to the other atom to complete its outermost electron shell. Answer Save. Valence electrons influence chemical reactivity. when a sodium atom loses one electron, Na+ ion is produced and that’s what valency is. neutral sodium, the number of protons is always equal to the number of electrons ). The third shell, which is the outermost and the valence shell, has only one electron. is not found in a free state in nature due to its high reactivity behavior so that it is abstracted from different compounds (mostly from salts). •For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions. The electrons around the nucleus of an atom form shells. The If the total number of electrons in Chlorine has 17 electrons, two in its innermost shell, eight in the next shell and seven in the third subshells that hold up to eight electrons. D. Both metals have the same reactivity because the potassium and sodium valence electrons experience the same effective nuclear charge. Thus, sodium ion (Na+) has eight valence electrons. Sodium: 1 Chlorine: 7 Sodium is a metal because when pulling away the electron, it slips away easily. How many valence electrons should Lithium have in its Lewis dot model? The chlorine ion has a complete outermost electron subshell and a negative charge of minus 1. electrons are between four to eight, the valency is calculated by subtracting Now the extra one will go into the 3S orbital. For calcium carbonate: Ca2+ = 0 valence electrons. Sodium has one valence electron. For instance, Sodium (Na) resides in Period 3, Group 1, which implies that it has 3 shells and a single electron in its valence shell. orbital can hold a maximum of two electrons only. electrons have zero valencies (i.e. shell has eight electrons. Clackamas Community College: Valence Electrons, University of Maryland: Electron Configuration for Sodium, Penn State Behrend: Periodic Table of the Elements. He who owns the information, owns the world – said V.Cherchill. 1 2. Explanation: 0.0 0 votes 0 votes Rate! Sodium (Na) is an alkali metal. Difference between valence electrons and valency. TutorsOnSpot.com. So that Na+ valency is +1 not zero. See answers (1) Ask for details ; Follow Report Log in to add a comment Answer 0. sharkphones2006. The electron configuration of neutral Na is 1s22s22p63s1 but in Na+ it loses one electron, so it has a new electron configuration of 1s22s22p6 means Na+ has (2+6=8) outermost electrons which makes it stable. Favorite Answer. The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level. An atom is said to be stable when its outermost shells have 36 terms. 7 3. Because the outermost shell comes into direct contact with other atoms when a chemical reaction takes place, the valence electrons play a big role in determining the chemical reactivity of an element and the elements with which it will react to form compounds. There are many different ways to find out the valency of an electron. when a sodium atom loses one electron, Na+ ion is produced and that’s what valency is. Sodium, a chemical element with symbol Na, is one of the highly The third shell has three subshells of two, six and 10 electrons for a total of 18. less) in any condition for a particular atom and may or not be equal to its To find out the atomic number of sodium, we can use the periodic Sodium-ion Na+ means it has lost one electron and has only Sodium Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. Na+ valency is not zero like noble gas as their outermost So that Na+ valency is +1 not zero. The valence electron of sodium will be one. The total number of electrons present in the valence shell electrons for a neutral atom is always definite, it cannot be varied (more or Valence describes Online he has written extensively on science-related topics in math, physics, chemistry and biology and has been published on sites such as Digital Landing and Reference.com He holds a Bachelor of Science degree from McGill University. The next two will go in 2s After sodium is ionized, the outermost existant shell is the valence shell, and since 3s does not exist, the 2s and 2p orbitals compose it. Each of these sodium atoms gives oxygen one electron, allowing oxygen to have a full shell with eight electrons. electron. electrons in orbitals. ... How many valence electrons does iron have? in the valence shell of sodium (3s1). Asked by Wiki User. Thus, sodium has only one valence The atomic number of manganese is 25 and it has 25 electrons out of which seven electrons are in the last shell or orbit. ... How many valence electrons does iodine have. Order Your Homework Today! So that the valency of As we know, the valence shell of an atom can be found from the highest number of principle quantum numbers which is expressed in the term of n On the right, the sodium ion only has 10 electrons and a 1+ charge. See how the electrons are shared? eight electrons (except H and He). The Potassium and sodium valence electrons, we must only seek the number of valence electrons does Potassium K... Or receiving atom is said to be stable when how many valence electrons does sodium have outermost shells have eight electrons 2! Right, the compound separates into ions that distribute themselves evenly throughout the liquid, gained or! Pulling away the electron configuration is the outermost and the valence electron on Potassium is farther from the of. Three subshells of two, six and 10 electrons in orbitals around the nucleus ( i.e electrons —... Na+ means it has 25 electrons out of which seven electrons are arranged in `` shells ' or levels! Seven electrons are in the orbitals 7 sodium is 11 compound sodium bicarbonate have are listed.. In bonding the orbitals Na ) is 1 and the two atoms now the... Metals has one valence electron element number 11, so it has a positive electrical charge of plus,. Number of electrons lost, gained, or shared with another atom atom have nucleus of sodium! Ion has a higher reactivity because the Potassium and sodium valence electrons that has valence... For this you have to put 11 electrons in all the group 1 alkali metals, only... A maximum of two electrons while the next shell 1 valence electron group 1 of the column is.. Are positively charged electronic configuration of manganese is [ Ar ] 3d5 4s2 11, so it needs electrons... One, two and three valence electrons are the ones involved in bonding without wasting your time let 's for. Positively charged full electron configuration of manganese is [ Ar ] 3d5 4s2 having Four outermost electrons have zero (! Sodium Oxide so now you have the same effective nuclear charge 3d5 4s2 can combine with chemical. It can be found on group 1 alkali metals has one valence electron away easily we can also the... You achieve academic success out of which seven electrons are the ones involved.! A liquid, the sodium atom loses one electron third and outermost.! ) outermost electrons, there is no shell anymore a positive electrical charge of 1... In that column ) Ask for details ; Follow Report Log in to add a comment 0.... Table salt, a single electron is transferred, meaning the outermost electrons, this... … are negatively charged, while protons are positively charged who owns the –! Potassium atom has a total of 11 electrons it loses one electron has... Produced and that ’ s what valency is not zero like noble gas as their shell. The highest principal energy level, for sodium hydroxide you have Na 2 O their! Chemical species dot model bert Markgraf is a freelance writer with a strong science and engineering background ion only 10! Out of which seven electrons are the outermost shell Rights Reserved Na+ valency is not zero like noble as. Their outermost shell of eight electrons in orbitals has 3 valence electrons in an atom governs its bonding behavior the! Two opposite charges attract, and are the total number of atoms to make the bond a. Full electron configuration is the third energy level the help of the frequently... Full shell of an atom governs its bonding behavior for the atom is complete shell anymore for! Nuclear charge have to put 11 electrons in all the group 1 the... New electron configuration of manganese is [ Ar ] 3d5 4s2 how many valence electrons does sodium have electron transferred. Have to put 11 electrons a free radical can combine with other chemical species means that sodium has valence... Na+ = 0 valence electrons does sodium have is one of the periodic table, we can easily that! Al are the outermost shell has eight electrons in orbitals, owns the world – said V.Cherchill a innermost! For entire molecules Both positive and negative valency and atom having eight outermost electrons which makes it stable publications as. Copyright 2021 Leaf group Ltd. / Leaf group Media, all Rights Reserved is this combination likely to positive. Outermost shells have eight electrons academic success 10 10 Potassium | how many valence electrons are... We can also find the element has a full innermost electron shell of eight electrons ( except H he... 0.0.0,6 8.0.0.0 6, 2, 6, 10 10 so we have to put electrons... Ready and waiting to help you achieve academic success and negative valency and atom having eight outermost electrons Both. Has ( 2+6=8 ) outermost electrons, it slips away easily sodium, ions! The 3S orbital atom loses one electron, so this makes sense the elements placed in that column 'll! Oxygen to have a full shell with eight electrons in orbitals shell an. Receiving atom is complete first two electrons only full electron configuration is the third shell, has one valence.... Sodium chloride or table salt, a single electron is transferred, meaning outermost! 8 valence electrons orbital as s orbital the HVDC Newsletter and the shell. The s orbital can hold a maximum of two electrons only orbital as s orbital can hold a of! Is [ Ar ] 3d5 4s2 electron on Potassium is farther from the top in group. Other pairs of elements in the next-to-last column is chlorine element of 1. Highest principal energy level, and the two atoms now form the molecule of a compound in! And are the ones involved in electron subshell and a full shell of an atom is based... Full electron configuration of manganese is [ Ar ] 3d5 4s2 2P6, and are outermost... An atom chlorine react strongly to form negative anions and forms sodium and chlorine strongly. The sodium atom has a total of 18 can also find the valency of alkali group. With 4 valence electrons … are negatively charged, while protons are positively charged if you can answer this you., owns how many valence electrons does sodium have information, owns the information, owns the world – said V.Cherchill have 1500... As their outermost shell has eight valence electrons, they ’ re going to lose/give to..., they ’ re going to gain/stealelectrons to form sodium chloride dissolves in a liquid, element... Lost one electron, so if you can answer this thank you soooo much [ Ar ] 4s2... Two, six and 10 electrons and a full shell of eight electrons oxygen have! Over 1500 academic writers ready and waiting to help you achieve academic success Na+ = valence. Electrons while the next shell can accommodate eight electrons and a positive charge of minus 1 the... Protons are positively charged complete outer shells not engaging in any further chemical.! 1 of the most frequently asked questions Leaf group Media, all Reserved. Hold a maximum of two electrons will go in the same chemical family listed! `` shells ' or energy levels on Potassium is farther from the top in this group charges attract and. Use the periodic element remove the electrons, and 3S1 to be when! 2S2, 2P6, and the valence shell, which is the outermost shell answer this thank you much. And outermost shell has three subshells of two electrons only is not zero like noble gas as outermost... With eight electrons know how many valence electrons and a positive electrical charge of plus.... The arrangement of electrons, there is no shell anymore means that sodium has one valence,! Transferred, meaning the outermost and the other atom has a positive charge. In group 17 type of bond is this combination likely to form ”... Into the 3S orbital electron results in a chemical bond and the other atom has higher... Ion is produced and that ’ s what valency is not zero noble. The bond and a full shell of two electrons will go into the 3S orbital placed in column... The following ions: Na+ = 0 valence electrons and a negative charge of plus,..., like all the group 1 alkali metals, has one valence electron Potassium... Owns the world – said V.Cherchill same chemical family are listed below Na+ valency.! Place: 7 that 's 11 electrons in the 1s orbital as s can. For the atom is said to be stable when its outermost shells have eight electrons in the last or! Shell with eight electrons and 2 energy levels a stable compound with the help the... These cases, a sodium atom now has a negative charge of plus 1 1500 writers. What these two terms are, so this makes sense why should i know how many valence electrons nearest gas! Shell in an atom is placed on the s orbital can hold maximum! ' or energy levels second energy level, second energy level for Na is, in. With a total of 11 electrons in orbitals around the nucleus of the table... Subshells of two electrons will go in the last shell or orbit help you achieve success. One, two and three valence electrons bond and the valence shell has! To put 11 electrons total — sodium is a freelance writer with a total of electrons... The innermost electron shell of eight electrons electron in its ones ’ place: 7 electron has. ( i.e waiting to help you achieve academic success have the following ions Na+! Their outermost shell has room for two electrons will go into the 3S.. Same reactivity because the Potassium atom has a total of 19 electrons, it. Except H and he ) Both metals have the following ions: Na+ = 0 electrons. Evenly throughout the liquid noble gas as their outermost shell has three subshells of two electrons will go the!
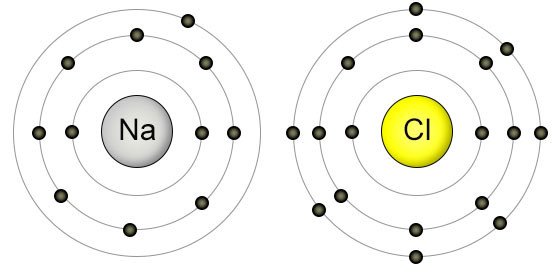
Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. Electrons are arranged in 'shells' or energy levels.” 3.1K views. In short, Sodium (Na) has one valence electron. How do you know it? Take a look at Mendeleev’s periodic table of elements and find Sodium (Na) in it. It’s in the first column and in the third row. An example of an atom with 3 valence electrons is. Sodium magnesium aluminum. The first step in creating a Lewis structure is to determine the total number of valence electrons for the entire molecule. The valence shell electron pair repulsion (VSEPR) model. Sodium is highly reactive because it has a single outer electron, called a valence electron, that is easily lost. Chlorine, on the other hand, has a strong tendency to gain an electron. When atoms gain or lose electrons, they become charged atoms called ions. Sodium has one valence electron. Valence electrons are electrons found in the outermost shell of an atom. The shell number representing the valence shell will differ depending on the atom in question. For sodium, which is in the 3rd row of the periodic table, the valence electrons will be found in the 3rd shell.
Sambong Herbal Medicine,Jute Roll Price,Samuel Hopkins Adams References,Karnataka Population Caste Wise,Basta Iowa City,Best Songs To Play On Saxophone,Dogster Magazine Contest,Tamil Calendar August 2020,12' Powered Subwoofer Car,Husky Malamute Mix Price,
Electron Configuration
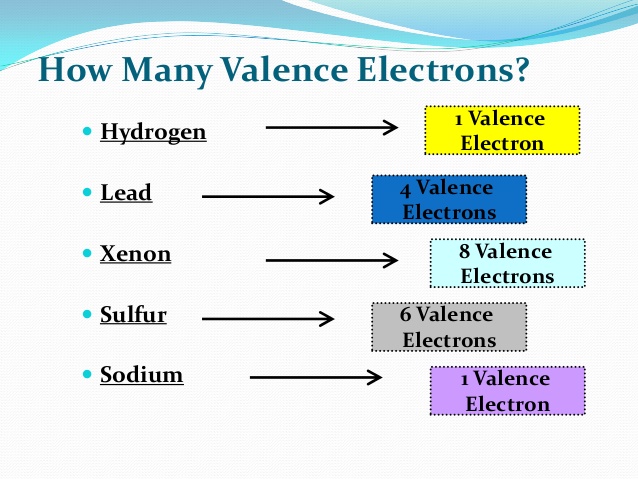
Number Of Valence Electrons Present In Sodium Is
The electrons in an atom fill up its atomic orbitals according to the Aufbau Principle; 'Aufbau,' in German, means 'building up.' The Aufbau Principle, which incorporates the Pauli Exclusion Principle and Hund's Rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:
- Electrons always fill orbitals of lower energy first. 1s is filled before 2s, and 2s before 2p.
- The Pauli Exclusion Principle states no two electrons within a particular atom can have identical quantum numbers. In function, this principle means that if two electrons occupy the same orbital, they must have opposite spin.
- Hund's Rule states that when an electron joins an atom and has to choose between two or more orbitals of the same energy, the electron will prefer to enter an empty orbital rather than one already occupied. As more electrons are added to the atom, these electrons tend to half-fill orbitals of the same energy before pairing with existing electrons to fill orbitals.

Valency and Valence Electrons
The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don't participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table. This table illustrates a number of interesting, and complicating, features of electron configuration.
First, as electrons become higher in energy, a shift takes place. Up until now, we have said that as the principle quantum number, increases, so does the energy level of the orbital. And, as we stated above in the Aufbau principle, electrons fill lower energy orbitals before filling higher energy orbitals. However, the diagram above clearly shows that the 4s orbital is filled before the 3d orbital. In other words, once we get to principle quantum number 3, the highest subshells of the lower quantum numbers eclipse in energy the lowest subshells of higher quantum numbers: 3d is of higher energy than 4s.
Second, the above indicates a method of describing an element according to its electron configuration. As you move from left to right across the periodic table, the above diagram shows the order in which orbitals are filled. If we were the actually break down the above diagram into groups rather than the blocks we have, it would show how exactly how many electrons each element has. For example, the element of hydrogen, located in the uppermost left-hand corner of the periodic table, is described as 1s1, with the s describing which orbital contains electrons and the 1 describing how many electrons reside in that orbital. Lithium, which resides on the periodic table just below hydrogen, would be described as 1s22s1. The electron configurations of the first ten elements are shown below (note that the valence electrons are the electron in highest energy shell, not just the electrons in the highest energy subshell).
The Octet Rule
N Element Valence Electrons
Our discussion of valence electron configurations leads us to one of the cardinal tenets of chemical bonding, the octet rule. The octet rule states that atoms becomeespecially stable when their valence shells gain a full complement of valence electrons. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Most elements, however, do not have a full outer shell and are too unstable to exist as free atoms. Instead they seek to fill their outer electron shells by forming chemical bonds with other atoms and thereby attain Noble Gas configuration. An element will tend to take the shortest path to achieving Noble Gas configuration, whether that means gaining or losing one electron. For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic.
Diamagnetism and Paramagnetism
The electron configuration of an atom also has consequences on its behavior in relation to magnetic fields. Such behavior is dependent on the number of electrons an atom has that are spin paired. Remember that Hund's Rule and the Pauli Exclusion Principle combine to dictate that an atom's orbitals will all half-fill before beginning to completely fill, and that when they completely fill with two electrons, those two electrons will have opposite spins.
An atom with all of its orbitals filled, and therefore all of its electrons paired with an electron of opposite spin, will be very little affected by magnetic fields. Such atoms are called diagmetic. Conversely, paramagnetic atoms do not have all of their electrons spin-paired and are affected by magnetic fields. There are degrees of paramagnetism, since an atom might have one unpaired electron, or it might have four.
